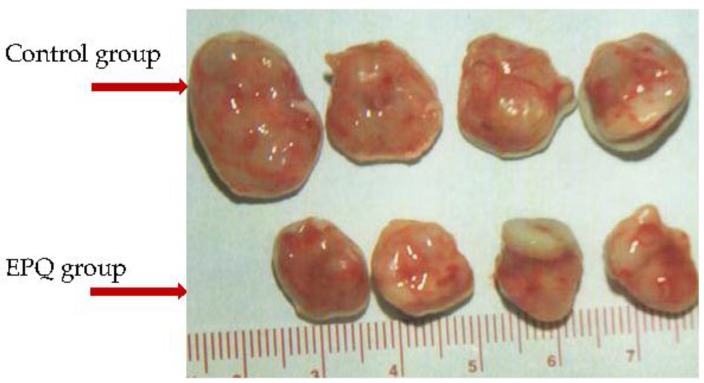
Los polifenoles, que se encuentran abundantemente en las plantas, muestran muchas propiedades anticancerígenas, incluyendo sus efectos inhibidores sobre la proliferación de células cancerosas, el crecimiento de tumores, la angiogénesis, la metástasis y la inflamación, además de inducir la apoptosis. Además, pueden modular la respuesta del sistema inmunológico y proteger las células normales contra el daño causado por los radicales libres. La mayoría de las investigaciones sobre los mecanismos anticancerígenos de los polifenoles se han realizado con compuestos individuales. Sin embargo, varios estudios, incluidos los nuestros, han indicado que la eficacia anticancerígena y el alcance de acción pueden mejorarse aún más al combinarlos sinérgicamente con compuestos químicamente similares o diferentes. Mientras que la mayoría de los estudios investigaron los efectos anticancerígenos de combinaciones de dos o tres compuestos, nosotros utilizamos mezclas más amplias de polifenoles específicos y mezclas de polifenoles con vitaminas, aminoácidos y otros micronutrientes. La mezcla que contiene quercetina, curcumina, té verde, cruciferex y resveratrol (PB) demostró una inhibición significativa del crecimiento de carcinoma de células escamosas de cabeza y cuello de anemia de Fanconi, e inhibición dosis-dependiente de la proliferación celular, la secreción de metaloproteinasas de matriz (MMP)-2 y -9, la migración celular y la invasión a través de Matrigel. Se encontró que PB era efectiva para inhibir la proliferación de células de fibrosarcoma HT-1080 y melanoma A2058, la expresión de MMP-2 y -9, la invasión a través de Matrigel y la inducción de apoptosis, parámetros importantes para la prevención del cáncer. Una combinación de polifenoles (quercetina y extracto de té verde) con vitamina C, aminoácidos y otros micronutrientes (EPQ) demostró una supresión significativa del crecimiento de tumores xenoinjertados de cáncer de ovario ES-2 y la supresión del crecimiento del tumor de ovario y metástasis pulmonar tras la inyección intraperitoneal (IP) de células de cáncer de ovario A-2780. La mezcla EPQ sin quercetina (NM) también ha mostrado potente actividad anticancerígena in vivo e in vitro en algunas decenas de líneas celulares de cáncer, inhibiendo el crecimiento tumoral y la metástasis, la secreción de MMP-2 y -9, la invasión, la angiogénesis y el crecimiento celular, así como la inducción de apoptosis. La presencia de vitamina C, aminoácidos y otros micronutrientes podría potenciar el efecto inhibidor del galato de epigalocatequina (EGCG) sobre la secreción de MMPs. Además, el enriquecimiento de NM con quercetina (mezcla EPQ) mejoró la actividad anticancerígena de NM in vivo. En conclusión, se ha demostrado que los polifenoles, especialmente en combinación con otros polifenoles o micronutrientes, son efectivos contra múltiples objetivos en el desarrollo y la progresión del cáncer, y deben considerarse como enfoques seguros y efectivos en la prevención y terapia del cáncer.
- Scalbert, A.; Manach, C.; Morand, C.; Rémésy, C.; Jime’nez, L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005, 45, 287–306. [CrossRef] [PubMed]
- Ramos, S. Cancer chemoprevention and chemotherapy: Dietary polyphenols and signaling pathways. Mol. Nutr. Food Res. 2008, 52, 507–526. [CrossRef] [PubMed]
- Fantini, M.; Benvenuto, M.; Masuelli, L.; Frajese, G.V.; Tresoldi, I.; Modesti, A.; Bei, R. In vitro and in vivo antitumoral effects of combinations of polyphenols, or polyphenols and anticancer drugs: Perspectives on cancer treatment. Int. J. Mol. Sci. 2015, 16, 9236–9282. [CrossRef] [PubMed]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jime’nez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [PubMed]
- Arts, I.C.W.; van de Putte, B.; Hollman, P.C.H. Catechin contents of foods commonly consumed in The Netherlands. Fruits, vegetables, staple foods, and processed foods. J. Agric. Food Chem. 2000, 48, 1746–1751. [CrossRef] [PubMed]
- Arts, I.C.; van de Putte, B.; Hollman, P.C.H. Catechin contents of foods commonly consumed in The Netherlands. Tea, wine, fruit juices, and chocolate milk. J. Agric. Food Chem. 2000, 48, 1752–1757. [CrossRef] [PubMed]
- Lakenbrink, C.; Lapczynski, S.; Maiwald, B.; Engelhardt, U.H. Flavonoids and other polyphenols in consumer brews of tea and other caffeinated beverages. J. Agric. Food Chem. 2000, 48, 2848–2852. [CrossRef] [PubMed]
- Zhu, Q.Y.; Zhang, A.Q.; Tsang, D.; Huang, Y.; Chen, Z.Y. Stability of green tea catechins. J. Agric. Food Chem. 1997, 45, 4624–4628. [CrossRef]
- Lempereur, I.; Rouau, X.; Abecassis, J. Genetic and agronomic variation in arabinoxylan and ferulic acid contents of durum wheat (Triticum durum L) grain and its milling fractions. J. Cereal Sci. 1999, 25, 103–110. [CrossRef]
- Bhat, K.P.; Pezzuto, J.M. Cancer chemopreventive activity of resveratrol. Ann. N. Y. Acad. Sci. 2002, 957, 210–229. [CrossRef] [PubMed]
- Adlercreutz, H.; Mazur, W. Phyto-oestrogens and western diseases. Ann. Med. 1997, 29, 95–120. [CrossRef] [PubMed]
- Toure, A.; Xuemin, X. Flax lignans: Source, biosynthesis, metabolism, antioxidant activity, bio-active ˙ components, and health benefits. Compr. Rev. Food Sci. Food Saf. 2010, 9, 261–269. [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [PubMed]
- Bogaards, I.J.P.; van Ommen, B.; Falke, H.E.; Willems, M.I.; van Bladeren, P.J. Glutathione S-transferase subunit induction patterns of Brussel sprouts, allyl isothiocyanate and goitrin in rat liver and small intestinal mucosa: A new approach for the identification of inducing xenobiotics. Food Chem. Toxicol. 1990, 28, 81–88. [CrossRef]
- Han, X.; Shen, T.; Lou, H. Dietary polyphenols and their biological significance. Int. J. Mol. Sci. 2007, 8, 950–988. [CrossRef]
- Shen, S.Q.; Zhang, Y.; Xiang, J.J.; Xiong, C.L. Protective effect of curcumin against liver warm ischemia/reperfusion injury in rat model is associated with regulation of heat shock protein and antioxidant enzymes. World J. Gastroenterol. 2007, 13, 1953–1961. [CrossRef] [PubMed]
- Chen, C.; Yu, R.; Owuor, E.D.; Kong, A.N. Activation of antioxidant response element (ARE), mitogen-activated protein kinases (MAPKs) and caspases by major green tea polyphenol components during cell survival and death. Arch. Pharm. Res. 2000, 23, 605–612. [CrossRef] [PubMed]
- Kunnumakkara, A.B.; Guha, S.; Krishnan, S.; Diagaradjane, P.; Gelovani, J.; Aggarwal, B.B. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor kappa B-regulated gene products. Cancer Res. 2007, 67, 3853–3861. [CrossRef] [PubMed]
- Collett, G.P.; Campbell, F.C. Curcumin induces c-jun N-terminal kinase-dependent apoptosis in HCT116 human colon cancer cells. Carcinogenesis 2004, 25, 2183–2189. [CrossRef] [PubMed]
- Anto, R.J.; Mukhopadhyay, A.; Denning, K.; Aggarwal, B.B. Curcumin (diferuloylmethane) induces apoptosis through activation of caspase-8, BID cleavage and cytochrome c release: Its suppression by ectopic expression of Bcl-2 and Bcl-xL. Carcinogenesis 2002, 23, 143–150. [CrossRef] [PubMed]
- Aoki, H.; Takada, Y.; Kondo, S.; Sawaya, R.; Aggarwal, B.; Kondo, Y. Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: Role of Akt and ERK signaling pathways. Mol. Pharmacol. 2007, 72, 29–39. [CrossRef] [PubMed]
- Anand, P.; Sundaram, C.; Jhurani, S.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin and cancer: An ‘old-age’ disease with an ‘age-old’ solution. Cancer Lett. 2008, 267, 133–164. [CrossRef] [PubMed]
- Alexandrov, M.G.; Song, L.J.; Altiok, S.; Gray, J.; Haura, E.B.; Kumar, N.B. Curcumin: A novel Stat3 pathway inhibitor for chemoprevention of lung cancer. Eur. J. Cancer Prev. 2012, 21, 407–412. [CrossRef] [PubMed]
- Ng, T.P.; Chiam, P.C.; Lee, T.; Chua, H.C.; Lim, L.; Kua, E.H. Curry consumption and cognitive function in the elderly. Am. J. Epidemiol. 2006, 164, 898–906. [CrossRef] [PubMed]
- Bartik, L.; Whitfield, G.K.; Kaczmarska, M.; Lowmiller, C.L.; Moffet, E.W.; Furmick, J.K.; Hernandez, Z.; Haussler, C.A.; Haussler, M.R.; Jurutka, P.W. Curcumin: A novel nutritionally derived ligand of the vitamin D receptor with implications for colon cancer. J. Nutr. Biochem. 2010, 21, 1153–1161. [CrossRef] [PubMed]
- Ide, H.; Tokiwa, S.; Sakamaki, K.; Nishio, K.; Isotani, S.; Muto, S.; Hama, T.; Masuda, H.; Horie, S. Combined inhibitory effect of soy flavones and curcumin on the production of prostate specific antigen. Prostate 2010, 70, 1127–1133. [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [CrossRef] [PubMed]
- Ravindran, J.; Prasad, S.; Aggrawal, B.B. Curcumin and cancer cells: How many ways can curry kill tumor cells selectively? AAPS J. 2009, 11, 495–510. [CrossRef] [PubMed]
- Shanmugam, M.K.; Rane, G.; Kanchi, M.M. The multifaceted role of curcumin in cancer prevention and treatment. Molecules 2015, 20, 2728–2769. [CrossRef] [PubMed]
- Gibellini, L.; Pinti, M.; Nasi, M.; Montagna, J.P.; De Biasi, S.; Roat, E.; Bertoncelli, L.; Cooper, E.L.; Cossarizza, A. Quercetin and cancer chemoprevention. Evid. Based Complement. Alternat. Med. 2011, 2011, 591356. [CrossRef] [PubMed]
- Ekstrom, A.M.; Serafini, M.; Nyren, O.; Wolk, A.; Bosetti, C.; Bellocco, R. Dietary quercetin intake and risk of gastric cancer: Results from a population-based study in Sweden. Ann. Oncol. 2011, 22, 438–443. [CrossRef] [PubMed]
- Lam, T.K.; Rotunno, M.; Lubin, J.H.; Wacholder, S.; Consonni, D.; Pesatori, A.C.; Bertazzi, P.A.; Chanock, S.J.; Burdette, L.; Goldstein, A.M.; et al. Dietary quercetin, quercetin-gene interaction, metabolic gene expression in lung tissue and lung cancer risk. Carcinogenesis 2010, 31, 634–642. [CrossRef] [PubMed]
- Jeong, J.H.; An, J.Y.; Kwon, Y.T.; Rhee, J.G.; Lee, Y.G. Effects of low dose quercetin: Cancer cell-specific inhibition of cell cycle progression. J. Cell. Biochem. 2009, 106, 73–82. [CrossRef] [PubMed]
- Yang, J.H.; Hsia, T.C.; Kuo, H.M.; Chou, P.D.; Chou, C.C.; Wei, Y.H.; Chung, J.G. Inhibition of lung cancer cell growth by quercetin glucuronides via G 2/M arrest and induction of apoptosis. Drug Metab. Dispos. 2006, 34, 296–304. [CrossRef] [PubMed]
- Nair, H.K.; Rao, K.V.K.; Aalinkeel, R.; Mahajan, S.; Chawda, R.; Schwartz, S.A. Inhibition of prostate cancer cell colony formation by the flavonoid quercetin correlates with modulation of specific regulatory genes. Clin. Diagn. Lab. Immunol. 2004, 11, 63–69. [CrossRef] [PubMed]
- Mu, C.; Jia, P.; Yan, Z.; Liu, X.; Li, X.; Liu, H. Quercetin induces cell cycle G1 arrest through elevating Cdk inhibitors p21 and p27 in human hepatoma cell line (HepG2). Methods Find. Exp. Clin. Pharmacol. 2007, 29, 179–183. [CrossRef] [PubMed]
- Seufi, A.M.; Ibrahim, S.S.; Elmagrahby, T.K.; Hafez, E.E. Preventive effect of the flavonoid, quercetin, on hepatic cancer in rats via oxidant/antioxidant activity: Molecular and histological evidences. J. Exp. Clin. Cancer Res. 2009, 28, 80. [CrossRef] [PubMed]
- Devipriya, S.; Ganapthy, V.; Shyamaladevi, C.S. Suppression of tumor growth and invasion in 9,10 dimethyl benz (a) anthracene induced mammary carcinoma by the plant bioflavonoid quercetin. Chem-Biol. Interact. 2006, 162, 106–113. [CrossRef] [PubMed]
- Theodoratou, E.; Kyle, J.; Cetnarskyj, R.; Farrington, S.M.; Tenesa, A.; Barnetson, R.; Porteous, M.; Dunlop, M.; Campbell, H. Dietary flavonoids and the risk of colorectal cancer. Cancer Epidemiol. Biomark. Prev. 2007, 16, 684–693. [CrossRef] [PubMed]
- Ferry, D.R.; Smith, A.; Malkhandi, J.; Fyfe, D.W.; de Takats, P.G.; Anderson, D.; Baker, J.; Kerr, D.J. Phase I clinical trial of the flavonoid quercetin: Pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin. Cancer Res. 1996, 2, 659–668. [PubMed]
- Miles, S.L.; McFarland, M.; Niles, R.M. Molecular and physiological actions of quercetin: Need for clinical trials to assess its benefits in human disease. Nutr. Rev. 2014, 72, 720–734. [CrossRef] [PubMed]
- Udenigwe, C.C.; Ramprasath, V.R.; Aluko, R.E.; Jones, P.J. Potential of resveratrol in anticancer and anti-inflammatory therapy. Nutr. Rev. 2008, 66, 445–454. [CrossRef] [PubMed]
- Bishayee, A. Cancer prevention and treatment with resveratrol: From rodent studies to clinical trials. Cancer Prev. Res. 2009, 2, 409–418. [CrossRef] [PubMed]
- Kukreja, A.; Waddhwa, N.; Tiwari, A. Therapeutic role of resveratrol and piceatannol in disease prevention. J. Blood Disord. Transf. 2014, 5, 1–6. [CrossRef]
- Patel, K.R.; Brown, V.A.; Jones, D.J.L.; Britton, R.G.; Hemingway, D.; Miller, A.S.; West, K.P.; Booth, T.D.; Perloff, M.; Crowell, J.A.; et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010, 70, 7392–7399. [CrossRef] [PubMed]
- Chow, H.H.S.; Garland, L.L.; Hsu, C.H.; Vining, D.R.; Chew, W.M.; Miller, J.A.; Perloff, M.; Crowell, J.A.; Alberts, D.S. Resveratrol modulates drug- and carcinogen-metabolizing enzymes in a healthy volunteer study. Cancer Prev. Res. 2010, 3, 1168–1175. [CrossRef] [PubMed]
- Drewnowski, A.; Gomez-Carneros, C. Bitter taste, phytonutrients and the consumer: A review. Am. J. Clin. Nutr. 2000, 72, 1424–1435. [PubMed]
- Hidgon, J.V.; Delage, B.; Williams, D.E.; Dashwood, R.H. Cruciferous vegetables and human cancer risk: Epidemiologic evidence and mechanistic basis. Pharmacol. Res. 2007, 55, 224–236.
- Holst, B.; Williamson, G. A critical review of the bioavailability of glucosinolates and related compounds. Nat. Proc. Rep. 2004, 21, 425–447. [CrossRef] [PubMed]
- Agerbirk, N.; Olsen, C.E. Glucosinolate structures in evolution. Phytochemistry 2012, 77, 16–45. [CrossRef] [PubMed]
- Kandala, P.K.; Srivastava, S.K. DIMming ovarian cancer growth. Curr. Drug Targets 2012, 13, 1869–1875. [CrossRef] [PubMed]
- Bradlow, H.L. Indole-3-carbinol as a chemopreventive agent in breast and prostate cancer. In Vivo 2008, 22, 441–446. [PubMed]
- Beaver, L.M.; Yu, T.W.; Sokolowski, E.I.; Williams, D.E.; Dashwood, R.H.; Ho, E. 3,30 -diindolylmethane, but not indole-3-carbinol, inhibits histone deacetylase activity in prostate cancer cells. Toxicol. Appl. Pharmacol. 2012, 263, 345–351. [CrossRef] [PubMed]
- Sepkovic, D.W.; Raucci, L.; Stein, J.; Carlisle, A.D.; Auborn, K.; Ksieski, H.B.; Nyirenda, T.; Bradlow, H.L. 3,30 -Diindolylmethane increases serum interferon-γ levels in the K14-HPV16 transgenic mouse model for cervical cancer. In Vivo 2012, 26, 207–211. [PubMed]
- Chinni, S.R.; Li, Y.; Upadhyay, S.; Koppolu, P.K.; Sarkar, F.H. Indole-3-carbinol (I3C) induced cell growth inhibition, G1 cell cycle arrest and apoptosis in prostate cancer cells. Oncogene 2001, 20, 2927–2937. [CrossRef] [PubMed]
- Li, Y.; Li, X.; Sarkar, F.H. Gene expression profiles of IC3 and DIM-treated PC3 human prostate cancer dells determined by cDNA microarray analysis. J. Nutr. 2003, 133, 1011–1019. [PubMed]
- Aggarwal, B.B.; Ichikawa, H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle 2005, 4, 1201–1215. [CrossRef] [PubMed]
- Sarkar, F.H.; Li, Y. Indole-3-carbinol and prostate cancer. J. Nutr. 2004, 134, 3493S–8349S. [PubMed]
- Plate, A.Y.; Gallaher, D.D. Effects of indole-3-carbinol and phenethyl isothiocyanate on colon carcinogenesis induced by azoxymethane in rats. Carcinogenesis 2006, 27, 287–292. [CrossRef] [PubMed]
- Tadi, K.; Chang, Y.; Ashok, B.T.; Chen, Y.; Moscatello, A.; Schaefer, S.D.; Schantz, S.P.; Policastro, A.J.; Geliebter, J.; Tiwari, R.K. 3,30 -Diindolylmethane, a cruciferous vegetable derived synthetic anti-proliferative compound in thyroid disease. Biochem. Biophys. Res. Commun. 2005, 337, 1019–1025. [CrossRef] [PubMed]
- Kim, Y.S.; Milner, J.A. Targets for indole-3-carbinol in cancer prevention. J. Nutr. Biochem. 2005, 16, 65–73. [CrossRef] [PubMed]
- Brew, C.T.; Aronchik, I.; Hsu, J.C.; Sheen, J.H.; Dickson, R.B.; Bjeldanes, L.F.; Firestone, G.L. Indole-3-carbinol activates the ATM signaling pathway independent of DNA damage to stabilize p53 and induce G1 arrest of human mammary epithelial cells. Int. J. Cancer 2006, 118, 857–868. [CrossRef] [PubMed]
- Chang, X.; Tou, J.C.; Hong, C.; Chen, Y.; Moscatello, A.; Schaefer, S.D.; Schantz, S.P.; Policastro, A.J.; Geliebter, J.; Tiwari, R.K. 3,30 -Diindolylmethane inhibits angiogenesis and the growth of transplantable human breast carcinoma in athymic mice. Carcinogenesis 2005, 26, 771–778. [CrossRef] [PubMed]
- Shukla, Y.; Kalra, N.; Katiyar, S.; Siddiqui, I.A.; Arora, A. Chemopreventive effect of indole-3-carbinol on induction of preneoplastic altered hepatic foci. Nutr. Cancer 2004, 50, 214–220. [CrossRef] [PubMed]
- Garikapaty, V.P.; Ashok, B.T.; Chen, Y.G.; Mittelman, A.; Iatropoulos, M.; Tiwari, R.K. Anti-carcinogenic and anti-metastatic properties of indole-3-carbinol in prostate cancer. Oncol. Rep. 2005, 13, 89–93. [CrossRef] [PubMed]
- Bonnesen, C.; Eggleston, I.M.; Hayes, J.D. Dietary indoles and isothiocyanates that are generated from cruciferous vegetables can both stimulate apoptosis and confer protection against DNA damage in human colon cell lines. Cancer Res. 2001, 61, 6120–6130. [PubMed]
- Keck, A.S.; Finley, J.W. Cruciferous vegetables: Cancer protective mechanisms of glucosinolate hydrolysis products and selenium. Integr. Cancer Ther. 2004, 3, 5–12. [CrossRef] [PubMed]
- Valcic, S.; Timmermann, B.N.; Alberts, D.S.; Wächter, G.A.; Krutzsch, M.; Wymer, J.; Guillén, J.M. Inhibitory effect of six green tea catechins and caffeine on the growth of four selected human tumor cell lines. Anticancer Drugs 1996, 7, 461–468. [CrossRef] [PubMed]
- Mukhtar, H.; Ahmad, N. Tea polyphenols: Prevention of cancer and optimizing health. Am. J. Clin. Nutr. 2000, 71, S1698–S1702, discussion S1703–S1704.
- Yang, G.Y.; Liao, J.; Kim, K.; Yurkow, E.J.; Yang, C.S. Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis 1998, 19, 611–616. [CrossRef] [PubMed]
- Taniguchi, S.; Fujiki, H.; Kobayashi, H.; Go, H.; Miyado, K.; Sadano, H.; Shimokawa, R. Effect of (−)-epigallocatechin gallate, the main constituent of green tea, on lung metastasis with mouse B16 melanoma cell lines. Cancer Lett. 1992, 65, 51–54. [CrossRef]
- Hara, Y. Green Tea: Health Benefits and Applications; Marcel Dekker: New York, NY, USA, 2001.
- Harakeh, S.; Abu-El-Ardat, K.; Diab-Assaf, M.; Niedzwiecki, A.; El-Sabban, M.; Rath, M. Epigallocatechin-3-gallate induces apoptosis and cell cycle arrest in HTLV-1-positive and -negative leukemia cells. Med. Oncol. 2008, 25, 30–39. [CrossRef] [PubMed]
- Cabrera, C.; Gimenez, R.; Lopez, M.C. Determination of tea components with antioxidant activity. J. Agric. Food Chem. 2003, 53, 4427–4435. [CrossRef] [PubMed]
- Fujiki, H.; Suganuma, M.; Okabe, S.; Sueoka, E.; Suga, K.; Imai, K.; Nakachi, K.; Kimura, S. Mechanistic findings of green tea as cancer preventive for humans. Proc. Soc. Exp. Biol. Med. 1999, 220, 225–228. [CrossRef] [PubMed]
- Gupta, S.; Hastak, K.; Afaq, F.; Ahmad, N.; Mukhtar, H. Essential role of caspases in epigallocatechin-3-gallatemediated inhibition of nuclear factor kappa B and induction of apoptosis. Oncogene 2004, 23, 2507–2522. [CrossRef] [PubMed]
- Ahmed, S.; Wang, N.; Lalonde, M.; Goldberg, V.M.; Haqqi, T.M. Green tea polyphenol epigallocatechin-3- gallate (EGCG) differentially inhibits interleukin-1 beta-induced expression of matrix metalloproteinase-1 and -13 in human chondrocytes. J. Pharmacol. Exp. Ther. 2004, 308, 767–773. [CrossRef] [PubMed]
- Khan, N.; Afaq, F.; Saleem, M.; Ahmad, N.; Mukhtar, H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006, 66, 2500–2505. [CrossRef] [PubMed]
- Ahn, W.S.; Yoo, J.; Huh, S.W.; Kim, C.K.; Lee, J.M.; Namkoong, S.E.; Bae, S.M.; Lee, I.P. Protective effects of green tea extracts (polyphenon E and EGCG) on human cervical lesions. Eur. J. Cancer Prev. 2003, 12, 383–390. [CrossRef] [PubMed]
- Kurbitz, C.; Heise, D.; Redmer, T.; Goumas, F.; Arlt, A.; Lemke, J.; Rimbach, G.; Kalthoff, H.; Trauzold, A. Epicatechin gallate and catechin gallate are superior to epigallocatechin gallate in growth suppression and anti-inflammatory activities in pancreatic tumor cells. Cancer Sci. 2011, 102, 728–734. [CrossRef] [PubMed]
- Kale, A.; Gawande, S.; Kotwal, S.; Netke, S.; Roomi, W.; Ivanov, V.; Niedzwiecki, A.; Rath, M. Studies on the effects of oral administration of nutrient mixture, quercetin and red onions on the bioavailability of epigallocatechin gallate from green tea extract. Phytother. Res. 2010, 24, S48–S55. [CrossRef] [PubMed]
- Wang, P.; Herber, D.; Henning, S.M. Quercetin increased bioavailability and decreased methylation of green tea polyphenols in vitro and in vivo. Food Funct. 2012, 3, 635–642. [CrossRef] [PubMed]
- Wang, P.; Herber, D.; Henning, S.M. Quercetin increased antiproliferative activity of green tea polyphenol (−)-epigallocatechin gallate in prostate cancer cells. Nutr. Cancer 2012, 64, 580–587. [CrossRef] [PubMed]
- Naumovsky, N.; Blades, B.L.; Roach, P.D. Food inhibits the oral bioavailability of the major green tea antioxidant epigallocatechin gallate in humans. Antioxidants 2015, 4, 373–393. [CrossRef] [PubMed]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S. [PubMed]
- Hsieh, T.C.; Wu, J.M. Targeting CWR22Rv1 prostate cancer cell proliferation and gene expression by combinations of the phytochemicals, EGCG, genistein and quercetin. Anticancer Res. 2009, 29, 4025–4032. [PubMed]
- Tang, S.N.; Singh, C.; Nall, D.; Meeker, D.; Shankar, S.; Srivastava, R.K. The dietary bioflavonoid quercetin synergizes with epigallocatechin gallate (EGCG) to inhibit prostate cancer stem cell characterstic, invasion migration and epithelial-mesenchymal transition. J. Mol. Signal. 2010, 5, 14. [CrossRef] [PubMed]
- Ahmad, K.A.; Harris, N.H.; Johnson, A.D.; Lindvall, H.C.; Wang, G.; Ahmed, K. Protein kinase CK2 modulates apoptosis induced by resveratrol and epigallocatechin gallate in prostate cancer cells. Mol. Cancer Ther. 2007, 6, 1006–1012. [CrossRef] [PubMed]
- Hsieh, T.C.; Wu, J.M. Suppression of cell proliferation and gene expression by combinatorial synergy of EGCG, resveratrol and gamma-tocotrienol in estrogen positive MCF-7 breast cancer cells. Int. J. Oncol. 2008, 33, 851–859. [PubMed]
- Saha, A.; Kuzuhara, T.; Echigo, N.; Suganuma, M.; Fujiki, H. New role of (−)epicatechin in enhancing the induction of growth inhibition and apoptosis in human lung cancer cells by curcumin. Cancer Prev. Res. 2010, 3, 953–962. [CrossRef] [PubMed]
- Zhou, D.H.; Wang, X.; Yang, M.; Shi, X.; Huang, W.; Feng, Q. Combination of low concentration of (−)epigallocatechin gallate (EGCG) and curcumin strongly suppresses the growth of non small cell lung cancer in vitro and in vivo through causing cell cycle arrest. Int. J. Mol. Sci. 2013, 14, 12023–12036. [CrossRef] [PubMed]
- Ghosh, A.K.; Kay, N.E.; Secreto, C.R.; Shanafelt, T.D. Curcumin inhibits prosurvival pathways in chronic lymphocytic leukemia B cells and may overcome their stromal protection in combination with EGCG. Clin. Cancer Res. 2009, 15, 1250–1258. [CrossRef] [PubMed]
- Somers-Edgar, T.J.; Scandlyn, M.J.; Stuart, E.C.; Le Nedelec, M.J.; Valentine, S.P.; Rosengren, R.J. The combination of epigallocatechin gallate and curcumin suppresses ER alpha-breast cancer cell growth in vitro and in vivo. Int. J. Cancer 2008, 122, 1966–1971. [CrossRef] [PubMed]
- Piao, L.; Mukherjee, S.; Chang, Q.; Xie, X.; Li, H.; Castellanos, M.R.; Banerjee, P.; Iqbal, H.; Ivancic, R.; Wang, X.; et al. TriCurin, a novel formulation of curcumin, epicatechin gallate and resveratrol, inhibits the tumorigenicity of human papillomavirus-positive head and neck squamous cell carcinoma. Oncotarget 2016. [CrossRef] [PubMed]
- Roomi, M.W.; Kalinovsky, T.; Roomi, N.W.; Niedzwiecki, A.; Rath, M. In vitro and in vivo inhibition of human Fanconi anemia head and neck squamous carcinoma by a phytonutrient combination. Int. J. Oncol. 2015, 46, 2261–2266. [CrossRef] [PubMed]
- Roomi, M.W.; Jariwalla, N.; Roomi, N.W.; Rath, M.; Niedzwiecki, A. Abstract 1500: A novel nutrient mixture exhibits antitumor activity in human fibrosarcoma cell line HT-1080. In Proceedings of the 102nd Annual Meeting of the AACR, Orlando, FL, USA, 2–6 April 2011.
- Roomi, M.W.; Siddiqui, S.; Roomi, N.W.; Niedzwiecki, A.; Rath, M. Abstract 1503: Anti-cancer effects of a nutrient mixture in human melanoma cells A2058: Inhibition of cell proliferation, MMP expression, invasion and apoptosis. In Proceedings of the 102nd Annual Meeting of the AACR, Orlando, FL, USA, 2–6 April 2011.
- Netke, S.P.; Roomi, M.W.; Ivanov, V.; Niedzwiecki, A.; Rath, M. A specific combination of ascorbic acid, lysine, proline and epigallocatechin gallate inhibits proliferation and extracellular matrix invasion of various human cancer cell lines. Res. Commun. Pharmacol. Toxicol. Emerg. Drugs 2003, 8, IV37–IV49.
- Stetler-Stevenson, W.G. The role of matrix metalloproteinases in tumor invasion, metastasis and angiogenesis. Surg. Oncol. Clin. N. Am. 2001, 10, 383–392. [PubMed]
- Dano, K.; Andreasen, P.A.; Grondahl-Hansen, J.; Kristensen, P.; Nielsen, L.S.; Skriver, L. Plasminogen activators, tissue degradation and cancer. Adv. Cancer Res. 1985, 44, 139–266. [PubMed]
- Rath, M.; Pauling, L. Plasmin-induced proteolysis and the role of apoprotein(a), lysine and synthetic analogs. Orthomol. Med. 1992, 7, 17–23.
- Park, S. The effects of high concentrations of vitamin on cancer cells. Nutrients 2013, 5, 3496–3505. [CrossRef] [PubMed]
- Chen, Q.; Espey, M.G.; Krishna, M.C.; Mitchell, J.B.; Corpe, C.P.; Buettner, G.R.; Shacter, E.; Levine, M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: Action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. USA 2005, 102, 13604–13609. [CrossRef] [PubMed]
- Lutsenko, E.A.; Carcamo, J.M.; Golde, D.W. Vitamin C prevents DNA mutation induced by oxidative stress. J. Biol. Chem. 2001, 277, 16895–16899. [CrossRef] [PubMed]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Micronutrient synergy—A new tool in effective control of metastasis and other key mechanisms of cancer. Cancer Metastasis Rev. 2010, 29, 529–543. [CrossRef] [PubMed]
- Roomi, M.W.; Kalinovsky, T.; Roomi, N.M.; Cha, J.; Rath, M.; Niedzwiecki, A. In vivo and in vitro effects of a nutrient mixture on breast 4T1 cancer progression. Int. J. Oncol. 2014, 44, 1933–1944. [PubMed]
- Roomi, M.W.; Monterrey, J.C.; Kalinovsky, T.; Rath, M.; Niedzwiecki, A. Comparative effects of EGCG, green tea, and a nutrient mixture on the patterns of MMP-2 and MMP-9 expression in cancer cell lines. Oncol. Rep. 2010, 24, 747–757. [PubMed]
- Kale, A.; Gawande, S.; Kotwal, S.; Netke, S.; Roomi, M.W.; Ivanov, V.; Niedzwiecki, A.; Rath, M. A combination of green tea extract, specific nutrient mixture and quercetin: An effective intervention treatment for the regression of N-methyl-N-nitrosourea (MNU)-induced mammary tumors in Wistar rats. Oncol. Lett. 2010, 1, 313–317. [PubMed]
- Roomi, M.W.; Kalinovsky, T.; Niedzwiecki, A.; Rath, M. A nutrient mixture modulates ovarian ES-2 cancer progression by inhibiting xenograft tumor growth and cellular MMP secretion, migration and invasion. Int. J. Clin. Exp. Med. 2016, 9, 814–822.
- Roomi, M.W.; Niedzwiecki, A.; Rath, M. Abstract 4053: A unique nutrient mixture suppresses ovarian cancer growth of A-2780 by inhibiting invasion and MMP-9 secretion. In Proceedings of the 107th Annual Meeting of the AACR, New Orleans, LA, USA, 16–20 April 2016.
- Wang, P.; Vadgama, J.V.; Said, J.W.; Magyar, C.E.; Doan, N.; Heber, D.; Henning, S.M. Enhanced inhibition of prostate cancer xenograft tumor growth by combining quercetin and green tea. J. Nutr. Biochem. 2014, 25, 73–80. [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/)